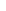팝업레이어 알림
팝업레이어 알림이 없습니다.Best Partner Your Business, Pharmsoft
The benefit of customers,
the benefit of partners,
and our profit.
COMPANY
Company Info.
We are an IT company that develops,
builds, and supports quality IT systems in the GxP area.
We were established in 2014 to develop a global quality IT system in line with
the Ministry of Food and Drug Safety's PIC/S subscription.
We continue to grow every year through new product development and product upgrades.
Our products are being developed in 8 categories (LIMS, ELN, LAS, QMS, EDMS, PQRS, LMS),
and all of them are implemented in the same framework environment
to maximize synergy through optimal interlocking between products.
Our program is being operated about 80 systems in 40 research institutes and factories pruducing drug,
raw material, biomedicine, medical device. It is now extended to the new product development process
and ISO operating work of the pharmaceutical company's headquarters.
Through continuous efforts, we want to grow into a leading software company in the regulatory-based industry.
OUR PARTNERSHIP
Partnership
BUSINESS
Business Area
-
Building a laboratory system
-
Establishment of quality
assurance system -
Atypical work management
Building a laboratory system
PharmLIMS/ELN/LAS/QMS
By building a LIMS system in the laboratory,, overall IT-ization of laboratory work is implemented. We systematize the entire process from test request to receipt, order, result review and approval, and management of samples, inventories such as reagents/standards, columns and equipment, and stability tests of pharmaceutical companies.
The ELN and LAS systems systemize the calculation sheets, logbooks and data that are not managed by the LIMS system. By automatically uploading the outputs and data of all test devices to the server and recording the necessary data in the calculation sheet and logbooks, you can dramatically reduce the number of post-test tasks and actively respond to Data Integrity issues. With the establishment of a QMS system linkage, it is possible to systemize lab inspection, OOS/OOT and to make paperless labs.
Establishment of quality assurance system
PharmEDMS/LMS/QMS/PQRS
With the introduction of PharmEDMS, we achieve breakthrough quality improvement and cost reduction by optimizing the process of preparing, reviewing, approval, training, release, distribution and collection of regulatory documents such as standards and SOPs, which are the basis of work. In particular, it is possible to perfectly respond to document-oriented due diligence by fundamentally preventing omission of work through linkage with the Education Management System (LMS).
While EDMS systematizes regulatory documents, PharmQMS systematizes the process of writing implementation documents. Real-time document creation, automatic list management and one-click tracking management are implemented by systematizing all documents and data for major quality assurance tasks such as management of change, deviation, CAPA, consumer complaints, validation and supplier evaluation. By using the Tablet PC to create various documents at the production site, it is possible to implement Paperless in connection with the MES system.
The Product Quality Assessment System (PQRS) collects all IT-based quality data and supports automatic report creation. It automatically collects data on all items such as production history, process yield, process inspection, test results, input raw materials, changes, deviations, validation status, and stability test. After automatically filling the data in the Excel form for each company, the necessary table forms and graphs are directly written in a word file. The person in charge writes a remark and sends the product quality evaluation report, and reviewers and approvers can also complete it through electronic signatures.
Atypical work management
PharmWFMS, Work Flow Management System, Work Flow Management System
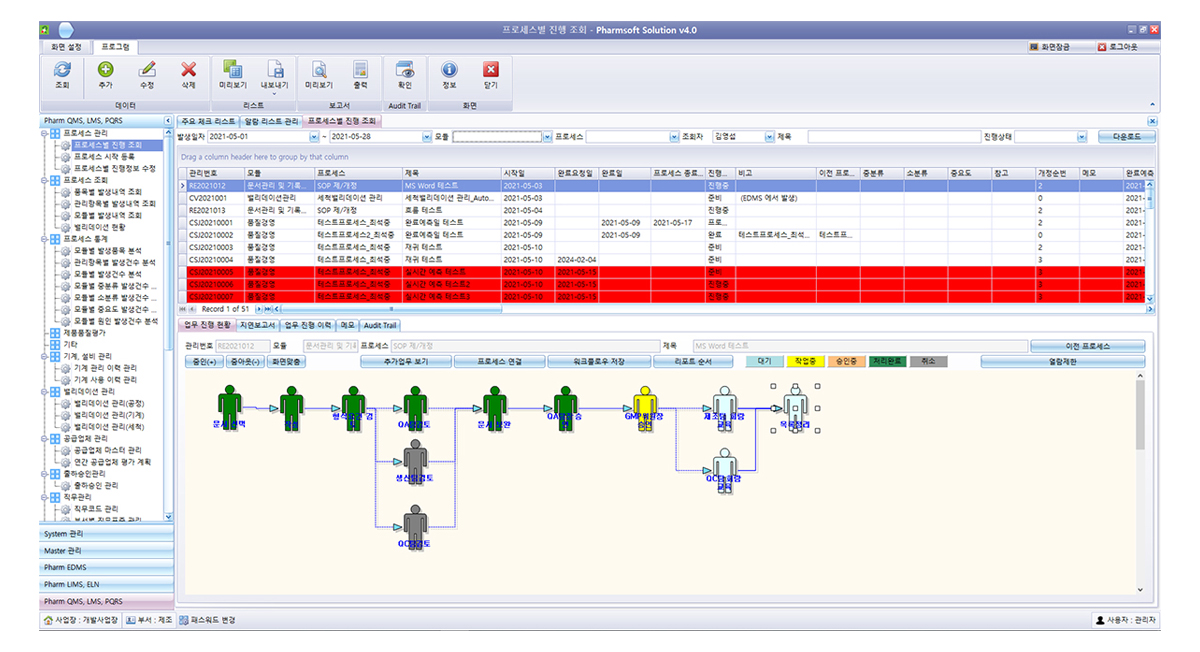
PharmWFMS is a system that allows you to set up your company's various tasks in a flowchart manner. The administrator can set it infinitely, and it runs according to the set sequence. It is used as a system to support the company's backbone system with excellent flexibility and is mainly used to support unstructured work and project-like work.
By applying it to the new product development process, it intuitively manages each stage from product review to release, communicating in real time with all relevant departments, and providing real-time statistics over several years.
By applying to ISO operation and project-related tasks, it can satisfy various tasks of each organization and maximize management efficiency.
PRODUCT
Products
PHARM PACKAGE
Pharm Package
Competitiveness

Comparison with domestic softwares
-
Packaged product
High quality, proven functions, many options, easy upgrades
-
Consulting skills
Separation of consulting team, overall understanding of GxP, third-party cases
-
Project skills
Stable performance according to standard implementation methodology

Comparison with global softwares
-
Convenience of use
Screen configuration that users can easily search/enter
-
Various functions
Provides various functions that help in real work
-
Upgrade
Upgrade support available every 1-2 months
-
Establishing/Operation Costs
Relative advantage of building cost and operating cost

Operation/Maintenance Aspects
-
Maintenance
Operation of a help page dedicated to maintenance.
Operation of a maintenance department -
Change Control
Support for CSV change control management
-
Extendability
Package function extension applied sequentially in various ways
product composition
QMS
Quality Assurance
Process
Statistical Analysis
EDMS
Document conversion
Document structure
Document publication
PQRS
Document form semi-automatic setting.
Data interworking
Document preparation
LMS
Job management
Education management
Individual history management
LIMS
Test report
Test records
Statistical analysis
ELN
Form setting
Log-Book
Equipment interworking
LAS
Device connection
Item extraction
Data transfer
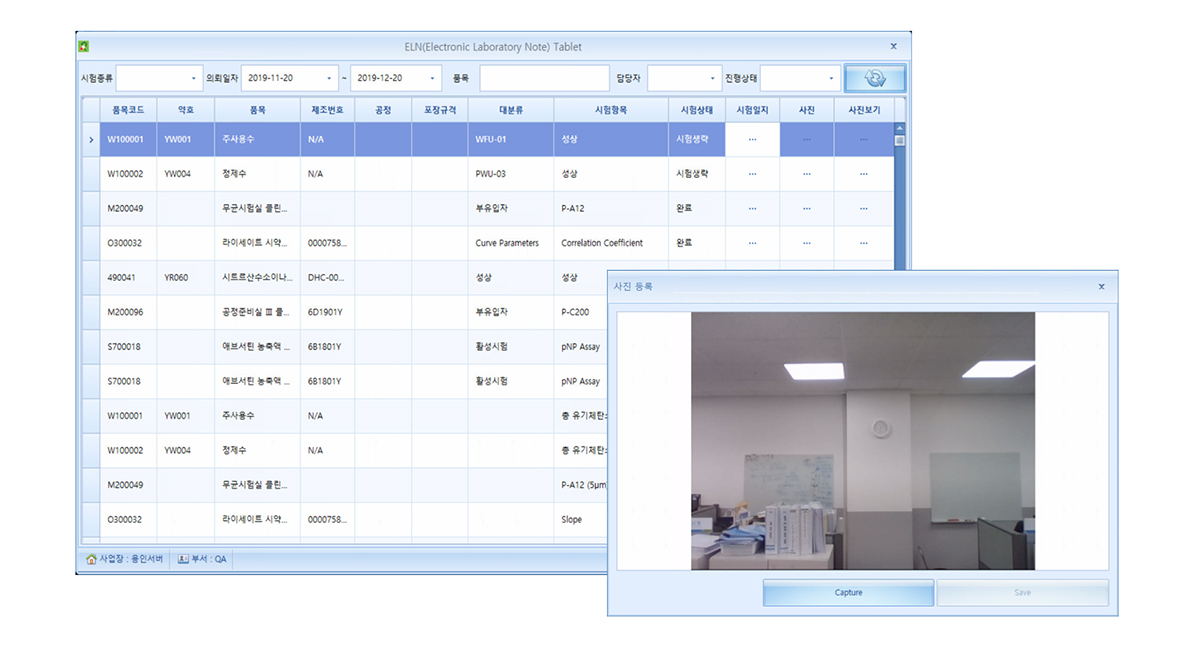
Tablet ELN
Test records log
Camera/Photo
Data transfer
Master Data Management
System & Configuration Management
Windows
MS SQL Server
- 게시물이 없습니다.